EXPeRT BRAINSTORMING AND PRODUCT DEFINITION
Transformation of an idea to concept then to a fully viable product. Throughout the engineering process using scientific principles, the design is refined. Within the constraints imposed by our client’s desires and physical reality, various optimization parameters are considered, such as, quality, volume, safety, and reliability..
EXPeRT IN MECHANICAL DESIGN
The design of elegantly simple products that have very clever mechanisms built into them the critical task is the integration of the sum of all the ideas into a functional system that fulfill the target requirements
My experience tells me where not to go and where to go, when to calculate and when to experiment.
DEVELOPER OF EARLY CONCEPTS TO FUNCTIONAL PROTOTYPE BUILD
I am experienced builder of different types of prototype technology, from cosmetic mockup to functional prototypes, working with my own equipment from 3D Printing and CNC machining and additionally work with a full network of vetted partners that together we provide processes like laser cutting, Sheetmetal, Quick turn PCBA, Silicone Molding..
DESIGN FOR MANUFACTURABILITY (DFM)
My designs are optimized for both performance and manufacturing cost. There are many ways to manufacture and assemble a product, and by applying DFM principles throughout the development process, I ensure that the final design is successful.
Experienced in these manufacturing processes
- injection molding, and extrusions of plastics
- Assembly process
- Diecasting
- Sheetmetal
- CNC Machining
- Joining and assembly methods
- Materials selection for your specific process and applications
- Dimensional tolerance of components applied to specific processes
IMPORTANCE OF DOCUMENTATION
document our design process per you requirements, experienced in EUMDR, FDA, ISO 13845 QMS FOR MED DEV, IEC 60601 MED DEV COMPLIANCE, ISO 14971 RISK MANAGEMENT, CFR 820 FDA QS CGMP.
Working with different customer requirements, furnishing documentation for their DHF, supporting 510k filling and amendments.
Following drafting standards, GD&T, Tolerance analysis, SPC, correlation of key performance parameters, SPC, and defining manufacturing documentation.
VERIFICATION AND VALIDATION
Experienced in development testing and verification testing through the the different phases of the project life cycle. developed test plans based on design inputs, tested and verified at critical milestones.
driven through complicated verification processes for Type II & III medical devices
Executed test protocols following good documentation processes, design of test fixtures from LABVIEW and MATLAB driven tests, DAQ recorded data, utilization of calibration practices, different sensors e.g. thermocouples, load cells, pressure sensors, flow meters, heat transfer with FLIR cameras and accelerated testing.
Development of traceability matrix to verify that design inputs linked to the verification tests to the verification report and ultimately confirmed compliance in a design review.
ABOUT ME
Thriving for an elegantly simple design that delivers the pricise function
A career of over twenty years of new product development and product delivery to manufacturing, where I managed, designed, and developed dozens of innovative new products across industrial, medical, and consumer markets.
Earned my BS in Mechanical Engineering from Universidad Iberoamericana in 1995. and received a Professional Certificate in Engineering Management from UCSD and additionally a Certificate in Product Strategy from Northwestern University in 2022.
Awarded multiple US patents. proven innovation facilitator and program manager, and have more than a decade of developing and documenting designs in a Medical device FDA/EUMDR environment.

Portfolio
Twenty plus years of product design and commercialization, that includes a decade of medical device experience.
Injection Molding part design
Sheet Metal
Diecast
Machining
Assembly process
Stamping
CAD Expert

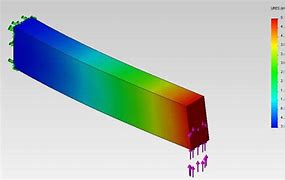
Experienced FEA
My work in numbers
Do you like my work?
A few things we’re great at
I challenge myself to be focused, organized, and to be analytical problem solver
MEDICAL DEVICE PRODUCT DEVELOPMENT
Developed concept to launch of Class II and Class III medical devices. From creating user requirements and handling regulatory compliance, to developing medical device designs, selecting materials, and participating with FDA submissions.
TEAM LEADERSHIP AND TECHNICAL DIRECTION
Team leader in many challenging projects, leadership by service and and trust, emphasis on empowering and inspiring teams to give the best of themselves .
- Understanding of my leadership style, traits and values .
- Mindful leadership built on resilience and adaptability.
- Experienced in building high-performing teams.
- Measured impact on my team and your organization .
BRAINSTORMING AND CONCEPT CREATION
Making innovation happen. with idea generation techniques leading a culture of collaboration to gather, manage, and implement impactful ideas that deliver value for your organization.
NEW PRODUCT INTRODUCTION INTO MANUFACTURING
Experienced in running an Idea, concept, design through the production ready phase while all rules and regulations are followed.
Client Testimonials
Don’t take our word for it – here’s what our clients say:
Luis designed our IVD consumables, he adapted to our team quickly and helped us define our design requirements and delivered an on target product.
Dequn (Daniel) Wang
CEO of Oranoxis Dx, San Diego CA
Luis is our go-to guy in the development of electronic enclosures, he is an expert in plastic injection molding and well connected to local and over-seas mold makers
James O’Shea
President at PDM Solutions, Inc San Diego Ca
Excellent engineering leader, he developed our tracking system enclosures and the mechanical verificatio
Carlos Rodriguez
resident and Founder of Bajatrack
Automated GPS logistics tracking systems
Contact me
I am ready and able to help: I provide design responsibility, monitored timeline, ultimately success through hard work!